Kaival Brands to Launch UK Market
GRANT, Fla. – Kaival Brands Innovations Group, Inc. (NASDAQ: KAVL), the global distributor of products manufactured by Bidi Vapor, LLC, announced its plan to launch distribution of its products in the United Kingdom.
Following the U.S. Food and Drug Administration’s decision to deny marketing authorization for flavored electronic nicotine delivery systems, which Bidi Vapor is challenging with respect to its BIDI® Stick, Kaival Brands and Bidi Vapor are excited to announce they are augmenting their focus on and attention to international markets where they have full market and product approvals to distribute the entire BIDI® Stick product line (including non-tobacco flavors).
While flavored ENDS face hurdles to get back on the U.S. market, the products remain legal in much of the world. Throughout the past twelve months, Bidi Vapor has received marketing and distribution approval in 11 global markets, including the United Kingdom and Russia. Given the favorable dynamics of the UK market (outlined below), Bidi Vapor and Kaival Brands are now focusing substantial time and strategic efforts on targeting the UK market. The Company believes that it has the budget to commit to marketing, staffing, and executing a successful strategy in the UK. Simultaneous to building out our UK infrastructure, the Company is actively discussing formalizing international distribution agreements with some of its current U.S. customers who also have global distribution capabilities.
THE UK VAPE MARKET: ATTRACTIVE FIT FOR BIDI VAPOR
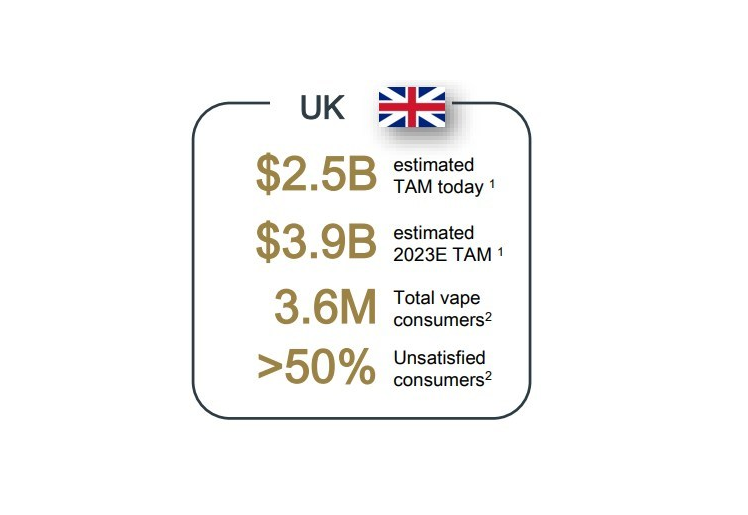
We believe the UK is an attractive market in which to launch our products internationally, with subsequent plans to roll out across the European Union, which represents a current ENDS market of $2.5 billion, and is expected to reach $3.9 billion by 2023.1
UK Market Snapshot:
- Mature market of adult consumers
- Average consumer age range of 35-45 years old, versus 18-24 in the United States
- Adult consumers (cigarette smokers) are motivated to transition to vape products2
Bidi Vapor created the BIDI® Stick to serve as a true, effective alternative for adult smokers of traditional combustible cigarettes, not as a product designed to attract first-time nicotine users. We believe that the UK market presents a strong fit as a market, as UK ENDS consumers are largely composed of current and former smokers, with under 3.5% of the total adult ENDS consumer population being represented by “never smokers.”
CONTINUED U.S. DOMESTIC MARKET OPPORTUNITY
The FDA received PMTAs covering over 6.5 million ENDS products from over 500 manufacturers prior to the September 9, 2020 court-ordered PMTA submission deadline. To date, the FDA has not approved a single PMTA for an ENDS product, and has rejected millions of applications for flavored ENDS. The FDA has indicated applications are still pending for review for tobacco and menthol ENDS; importantly, we believe PMTAs outstanding for closed-system tobacco and menthol ENDS are representative of 10 or fewer companies, making Bidi Vapor one of a select group. As such, while Bidi Vapor is hopeful that the courts will grant relief in its marketing denial challenge, either way we anticipate Bidi Vapor will eventually receive authorization for its tobacco and menthol BIDI® Sticks. While the FDA’s recent actions with respect to PMTAs have likely eliminated a significant number of suppliers to the ENDS market, we believe that the actions will have minimal impact on adult consumer demand for ENDS products. In fact, we view the dynamics of the U.S. ENDS market to be more favorable to Bidi Vapor after the PMTA decision process than before. Prior to the recent PMTA update, there were hundreds of companies competing in the marketplace. Now, a much smaller number of larger companies with sufficient resources to develop the scientific evidence needed to meet the FDA’s high public health protection standard have a chance to complete the rigorous PMTA process for tobacco and menthol ENDS
BLUE SKIES AHEAD
We have a fortified balance sheet and are laser-focused on resuming our revenue growth. Our near-term focus will be on the growth opportunities that we see in the UK and Europe, and having obtained necessary marketing and distribution approvals outside of the U.S. in the past year, we believe that we are now in a strong position in regards to executing our international plan. In the U.S., with reduced competition, we intend to reclaim our dominant market share in the U.S. for non-flavored vape products and anticipate enjoying higher visibility of orders as well as higher order price points.
“We have weathered two storms: COVID-19 and the FDA’s universal decision on flavored ENDs. These events had negative consequences for our business, but we believe the storm is over. After the rain, the sun comes out. We are well capitalized, and it is my belief we have the best ENDS product on the market for adult consumers. Our goals are to have a strong first year in the UK and to recapture our leading market share in ENDS in the U.S.,” notes Kaival Brand’s Founder & CEO, Niraj Patel.
Forward Looking Statements
This press release includes statements that constitute “forward-looking statements” within the meaning of federal securities laws, which are statements other than historical facts that frequently use words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “position,” “should,” “strategy,” “target,” “will,” and similar words. All forward-looking statements speak only as of the date of this press release. Although we believe that the plans, intentions, and expectations reflected in or suggested by the forward-looking statements are reasonable, there is no assurance that these plans, intentions, or expectations will be achieved. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecasted in such statements. Our business may be influenced by many factors that are difficult to predict, involve uncertainties that may materially affect results, and are often beyond our control. Factors that could cause or contribute to such differences include, but are not limited to, our near-term plans for focusing on the European markets; the timing and results of the FDA’s PMTA process; the scope of future FDA enforcement of regulations in the ENDS industry; the FDA’s approach to the regulation of synthetic nicotine and its impact on our business; the duration and scope of the COVID-19 pandemic and impact on the demand for the products we distribute; the actions governments, businesses, and individuals take in response to the pandemic, including mandatory business closures and restrictions on onsite commercial interactions; the impact of the pandemic and actions taken in response to the pandemic on global and regional economies and economic activity; the pace of recovery when the COVID-19 pandemic subsides; general economic uncertainty in key global markets and a worsening of global economic conditions or low levels of economic growth; the effects of steps that we could take to reduce operating costs; our inability to generate and sustain profitable sales growth; circumstances or developments that may make us unable to implement or realize anticipated benefits, or that may increase the costs, of our current and planned business initiatives; changes in government regulation or laws that affect our business; significant changes in our relationships with our distributors or sub-distributors; and those factors detailed by us in our public filings with the Securities and Exchange Commission. All forward-looking statements included in this press release are expressly qualified in their entirety by such cautionary statements. Except as required under the federal securities laws and the Securities and Exchange Commission’s rules and regulations, we do not have any intention or obligation to update any forward-looking statements publicly, whether as a result of new information, future events, or otherwise.
(This information is primarily sourced from Kaival Brands. Highly Capitalized has neither approved nor disapproved the contents of this news release. Read our Disclaimer here).